Table of Contents
In this series of articles, Will Chu, Science Editor for NutraIngredients, discusses some of the key issues and challenges facing the nutraceutical and food ingredient industry today.
Consumer interest in food and nutrition products offering a health impact continues to grow. By some estimates, for example, the global prebiotics ingredients market may reach $9.4 billion by 2026, increasing 8.7% annually between 2021 and 2026 (Industry ARC). In the face of this strong demand, food and ingredients manufacturers are taking a closer look at human milk oligosaccharides (HMOs). Alwine Kardinaal, Expertise Group Leader at NIZO, walks us through the possible health benefits and applications of these promising prebiotic compounds.
Will Chu: What are human milk oligosaccharides, and how are they produced?
Alwine Kardinaal: HMOs are non-digestible carbohydrates, and the third most abundant component of human breast milk, after lipids and lactose. They are similar to oligosaccharides and prebiotics from other sources, which are known to promote a healthy gut microbiome and immune system.
Given that human milk is our only source of nutrition as babies, the fact that HMOs are such a large component of it underscores their importance for our health, at least at that early age. This then opens up the question of whether they could also deliver a health impact for adults, or for specific consumer groups such as the elderly.
Human milk is the only source of significant amounts of naturally occurring HMOs. However, commercially viable amounts of 2′-Fucosyllactose (2′-FL) and Lacto-N-neotetraose (LNnT) are now being produced via microbial fermentation. These synthetic versions are already used in infant formulas, and are available for some other applications. Although the full picture of the role of HMOs in human milk isn’t yet understood, adding them to infant formula helps make the product more similar to breast milk in both composition and physiological effect.
While 2′-FL and LNnT are two of the most abundant HMOs in human milk, other synthetic HMOs are beginning to be produced as well. There are over 200 natural HMO compounds, with a lot of variation in structure, concentration and composition. But we don’t know which ones may offer a specific health impact, for infants or for adults.
WC: How do HMOs work, and what health benefits might they offer?
AK: HMOs are indigestible by the human body. When they reach the colon, they are broken down by the microbiota there. Bifidobacterium spp. and Bacteroides spp. both produce enzymes that are particularly effective at this. The HMOs can then be consumed by other ‘good’ gut bacteria, helping to promote gut health. HMOs also stimulate the gut bacteria to produce short chain fatty acids, which strengthen the gut’s mucous membrane. This mucosal barrier prevents ‘undesirable’ substances from being absorbed into the body, while letting nutrients pass.
Furthermore, like other prebiotics, HMOs may support immune function. They can help increase pathogen recognition (part of the innate immune response) and may also provide antimicrobial and antiviral activity. For example, by acting as receptors for potential pathogenic bacteria and viruses, they can stop these organisms from adhering to host cells and tissues, thereby preventing harm to the infected person.
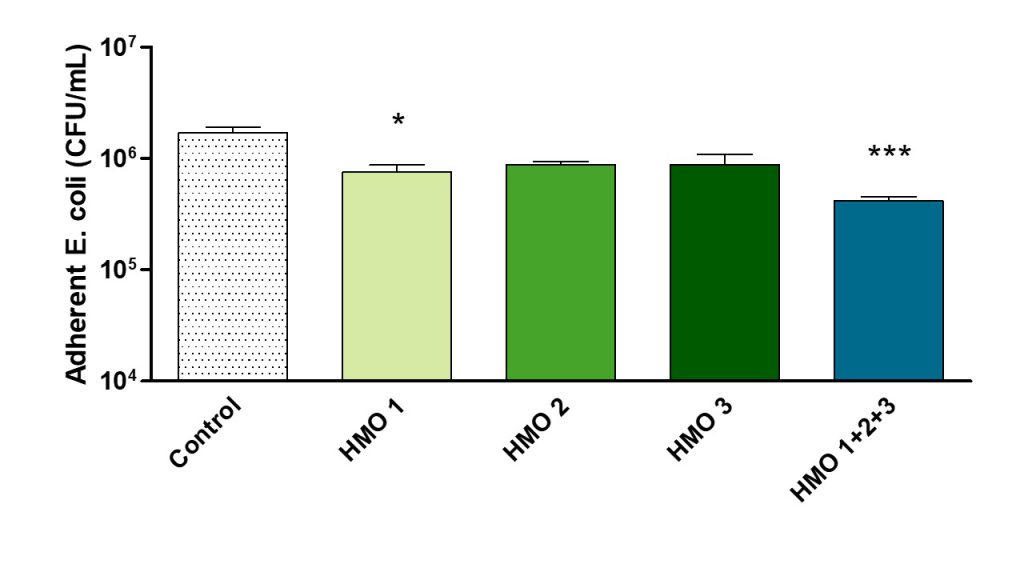
Tests of three HMOs showed that one significantly inhibited E. coli adhesion, and a combination of three had an additive effect.
WC: What is the scientific evidence for the health benefits of HMOs?
AK: There is strong in vitro evidence of the mechanistic effects of the HMOs. In vitro assays have demonstrated that HMOs impact gut microbiota composition, barrier function and immune modulation. For example, in vitro models mimicking fermentation in the gut show that HMOs increase the number of Bifidobacteria cells, which offer a number of health benefits.
There is also clinical evidence of the impact of HMOs on infants, especially 2′-FL and LNnT, which are currently the only compounds approved for use. These studies have shown that the HMOs are safe and well-tolerated, and that infants fed a formula enriched with the HMOs have lower inflammatory cytokines, fewer (parent-reported) respiratory infections, and a stool consistency and microbiota more similar to breast-fed infants. But there are still many questions: for example, one large study found no difference between infants who were fed formula with or without HMOs in terms of the number of respiratory tract infections or the duration of gastrointestinal infections.
For adults, the picture is even less clear, especially as so few studies have been done. Furthermore, the existing studies can have conflicting results. One pilot study on patients with irritable bowel syndrome (IBS) showed an effect from 2′-FL on gastrointestinal symptoms, faecal microbiota and short chain fatty acids, while an open-label, single arm study (a simple study design in which the volunteers were aware of the treatment being given) also showed improvement in bowel function and IBS symptoms. However, in a randomised control study, there was an increase in Bifidobacterium species, but no change in gastrointestinal symptoms.
WC: What is the current regulatory status for HMOs?
AK: 2′-FL and LNnT are labelled in the US as ‘Generally Recognised as Safe’ (GRAS) for use in infant formula and certain other foods and beverages. They are also approved as ‘Novel Foods’ in the EU and Canada, and are authorised for use in some Asian countries. Another HMO compound, 3′-Sialyllactose (3′-SL) has been submitted for GRAS notification, and has received a positive response from the FDA.
WC: What new developments and possibilities can we expect to see?
AK: After decades of research and development, we finally have adequate sources of synthetic HMOs both for testing and, ultimately, commercialisation. Beyond infant formula, other potential applications could include meal replacement or nutrition drinks or bars for adults and the elderly, and digestive health products.
At the same time, new types of synthetic HMO compounds are beginning to emerge. However, randomised control studies are needed to provide evidence of any health benefits.
The availability of synthetic HMOs is also enabling manufacturers to explore whether combining certain HMO compounds with other HMOs or probiotics, for example, might have a synergistic effect on gut health or immune function. Possible mixtures could first be screened in vitro, perhapsto assess their potential effect on the gut microbiome composition and functionality, or on immune modulation.
Clearly, HMOs offer enormous potential for ingredient and food manufacturers, but further research – especially clinical studies with adults – needs to be undertaken to provide scientific substantiation of the health benefits.
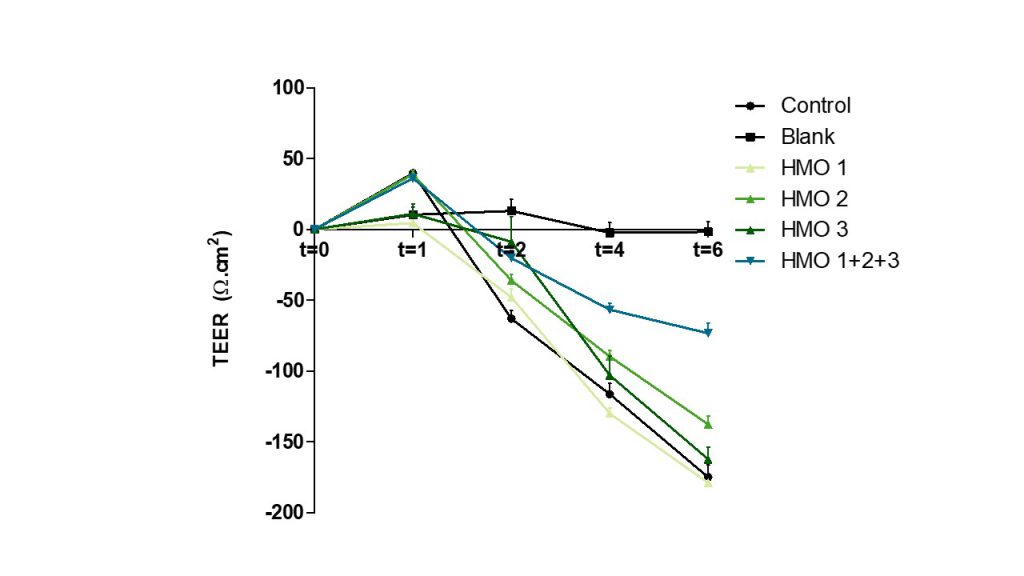
Testing has indicated a potential synergistic effect of combining 3 HMOs on preventing an E. coli- induced breach of the intestinal barrier
Cases
Our latest Blogs
© NIZO 2025 | Sitemap - Privacy Statement - Cookie Statement - Terms & Conditions
Website by: Online Marketing Agency